Summary
- Diplodia pinea diseases
- Pitch canker of pines
- Chrysoporthe (Cryphonectria) canker of eucalypts
- Botryosphaeria canker and die-back of eucalypts and black wattle
- Holocryphia (Endothia) canker of eucalypts
- Coniothyrium canker of eucalypts
- Pink disease of eucalypts
- Cytospora (Valsa) canker of eucalypts
- Bacterial wilt of eucalypts
- Black butt disease of wattle
- Ceratocystis wilt of wattle
Diplodia pinea diseases
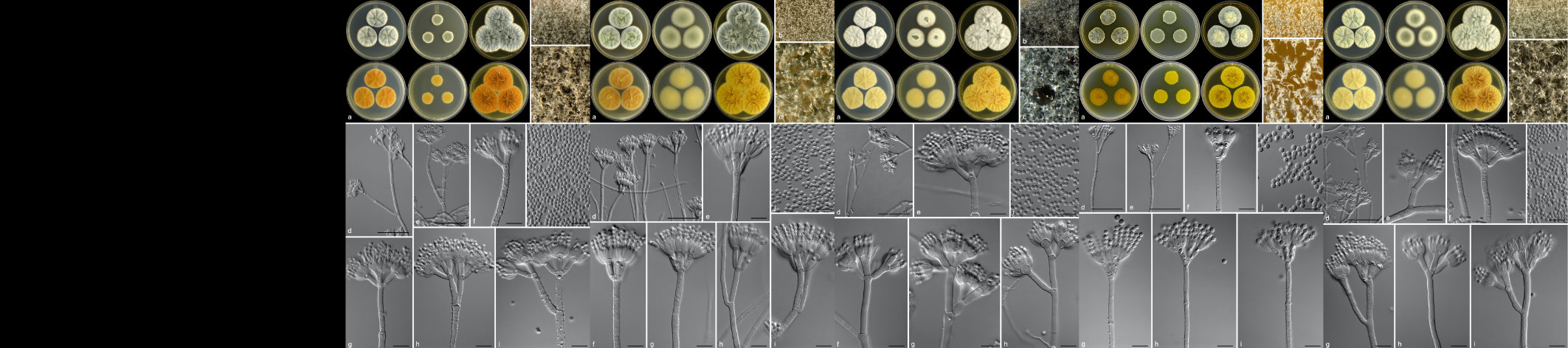
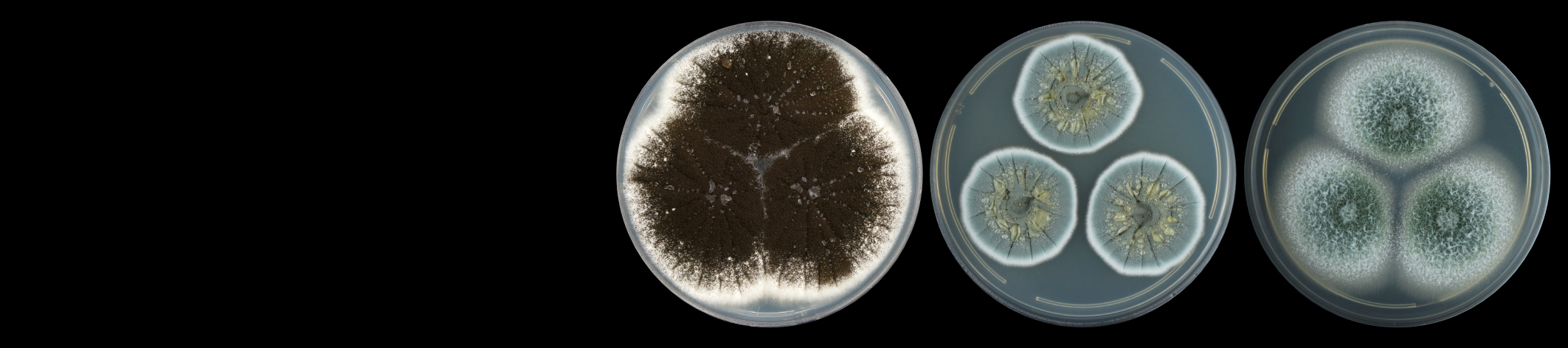
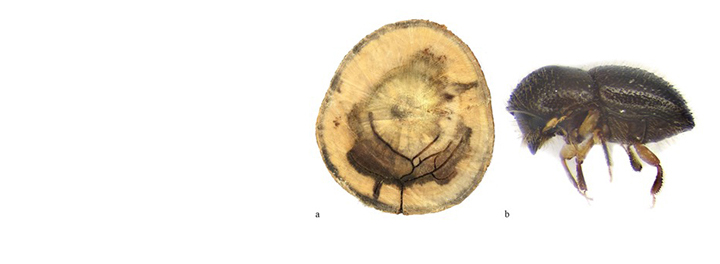
Diplodia pinea (Botryosphaeriaceae) is one of the most important pathogens of pines in South Africa. The fungus has been associated with a number of different disease symptoms, including shoot blight of seedlings and trees (die-back), stem cankers, root diseases and blue stain of timber (Wingfield and Knox-Davies 1980; Swart et al., 1987; Slippers and Wingfield 2007).
Diplodia pinea die-back following hail damage has been known in South Africa since the early 1900's. This serious disease, which accounts for the notoriety of the pathogen worldwide, has led to the restriction of the most susceptible species (Pinus patula, P. pinaster and P. radiata) to areas where hail is infrequent. Pinus patula is still planted in summer rainfall areas and severe losses commonly occur. Although less frequent, hailstorms do occur in areas where P. radiata is planted and these often also lead to substantial losses.
Diplodia pinea exists as a saprophyte on dead bark, cones and needles and is best known as an opportunist that infects wounds on susceptible pines. The fungus can, however, behave as a primary pathogen and infect young, unwounded pine shoots. Moisture is necessary for infection by D. pinea and young shoots become infected when rains coincide with warm temperatures, at the onset of growth. Once established in the shoots, D. pinea moves down the pith, where it can remain dormant. When pines are stressed, the fungus moves out of the pith and causes stem cankers.
A serious root disease of P. elliottii and P. taeda caused by D. pinea has been found in many parts of South Africa. Characteristic symptoms of this disease are dark-blue, radial lesions in young roots which extend to larger roots and into the trunk of diseased trees (Wingfield and Knox-Davies 1980). Needles become chlorotic and are shed. Pinus taeda is apparently most susceptible and the disease is always associated with stress due to factors such as overstocking, drought and plantings on poor sites.
Pitch canker of pines
Pitch canker of pines, caused by the fungus Fusarium circinatum (sexual state Gibberella circinata), has been known in the United States since the early 1900's. In that part of the world, it has caused occasional, yet serious damage to Pinus elliottii, while in California, Pinus radiata, has been seriously damaged by the fungus. Today, the fungus is known to cause nursery problems of Pinus spp. in Spain, Italy, Chile and South Africa, while reports have also been received from Japan.
The pitch canker pathogen was found for the first time in South Africa in the late 1980's where it caused a devastating root disease of P. patula seedlings in a commercial forest nursery in Mpumulanga. The pathogen is believed to have been introduced into the country on contaminated pine seed originating in Central America. The pathogen spread to all commercial pine nurseries within 10 years and is currently the most important nursery disease of Pinus spp. in the country, resulting in losses of up to 50% of P. patula seedlings during epidemics.
Significant losses, of especially P. patula, are experienced annually during establishment, as a result of plants dying from F. circinatum infection shortly after transplanting. It has been calculated that ~40% of plants moving to the field are asymptomatic, but develop disease soon after transplanting, resulting in high levels of blanking. According to a recent survey, 75% more seedlings are required to establish a P. patula stand as a result of F. circinatum infection of plants (Andrew Morris, Sappi 2010).
In recent years pitch canker disease on mature trees has been found in the Western, Southern and North Eastern Cape Provinces. The disease has resulted in the death of P. radiata, P. patula and P. greggii. It has also been shown to be associated with damage to trees caused by the weevil Pissodes nemorensis (Coutinho et al. 2007).
Chrysoporthe (Cryphonectria) canker of eucalypts
Chrysoporthe canker of eucalypts in South Africa is caused by the fungus Chrysoporthe austroafricana. The same disease in South America and Asia is caused respectively by Chr. cubensis (previously known as Cryphonectria cubensis) and Chr. deuterocubensis (previously known as C. cubensis). Although once considered the most serious disease of eucalypts in the world, the disease in many countries has been managed successfully through aggressive breeding and selection programmes and the deployment of disease tolerant clones (Gryzenhout et al., 2009).
In South Africa, disease of eucalypts caused by Chr. austroafricana is restricted to sub-tropical areas of the country. Although it commonly causes basal cankers on large trees, the most serious damage is to young E. grandis that become girdled at the base. Symptoms of the disease on young trees are a general wilting, and trees dying from Cryphonectria canker can easily be confused with those infected with Pythium sp. in Zululand. It is usually possible to identify trees infected with Chr. austroafricana by the presence of long-necked dark pycnidia (flask-shaped structures that produce and contain asexual spores) with orange spore masses at their apices, on the surface of the cankers. These can, however, be absent owing to unfavourable conditions for their production or because they have been consumed by insects. As is true in the case of most diseases, laboratory diagnosis is usually necessary.
Both Chr. cubensis and Chr. deuterocubensis have been introduced to the African continent, but as yet have not been found in South Africa. Chrysoporthe deuterocubensis, however, has been identified in central Mozambique and it is likely only a matter of time before it, or Chr. cubensis, appears in the country. The consequences of the introduction of these species into the country is not clear, although studies to date suggest that they are not as virulent as Chr. austroafricana. Care should, however, be taken not to move infected material into the country.
Botryosphaeria canker and die-back of eucalypts and black wattle
Fungi classified in the Botryosphaeriaceae are commonly associated with disease symptoms on eucalypts, Acacia mearnsii and Pinus spp. In the previous century, the disease of eucalypts and wattle was ascribed to Botryosphaeria dothidea. Research has, however, shown that Botryosphaeria canker is associated with numerous fungal species within a larger group, known as the Botryosphaeriaceae. This includes species of Neofusicoccum, Lasiodiplodia and Botryosphaeria.
Fungi in the Botryosphaeriaceae cause diseases characterized by stem cankers, shoot and branch die-back as well as root and root collar disease. Indeed, some of the most serious losses in plantations of eucalypts (and wattle) have been associated with the Botryosphaeriaceae. These fungi are opportunistic pathogens (often with a latent asymptomatic phase) and their importance is often underestimated. In terms of South African forestry, this fact is well illustrated by the importance of the closely related and opportunistic D. pinea on pines. Factors associated with infection of eucalypts and black wattle by fungi in the Botryosphaeriaceae include damage to growing tips by hot and cold winds or late frost, mechanical damage through hail, insect feeding or pruning and drought stress. One or more of these conditions commonly occur in local plantations and it is not surprising that extensive losses have been associated with these fungi (Slippers and Wingfield 2007).
Holocryphia (Endothia) canker of eucalypts
Holocryphia eucalypti (previously known as Endothia gyrosa and Cryphonectria eucalypti) causes a bark canker disease of eucalypts in many parts of South Africa and Swaziland (Gryzenhout et al., 2009). The pathogen is common on most eucalypt species in the country, causing superficial cracking of the bark. In some cases, however, the pathogen appears to penetrate the bark and infect the cambium. Infection of eucalypts appears to be dependent on two factors that can be, but are not necessarily, related. These include stress and inherent susceptibility of the trees. Cankers are most serious in areas affected by drought stress. However, certain species and clones of E. grandis, planted on what are considered to be excellent sites, can also be severely affected.
In Australia, H. eucalypti has been associated with the death of eucalypts, and is considered an important pathogen of these trees. Australia is believed to be the origin of H. eucalypti and it has been shown that the fungus in South Africa is represented by a limited diversity, indicative of an introduced pathogen.
Coniothyrium canker of eucalypts
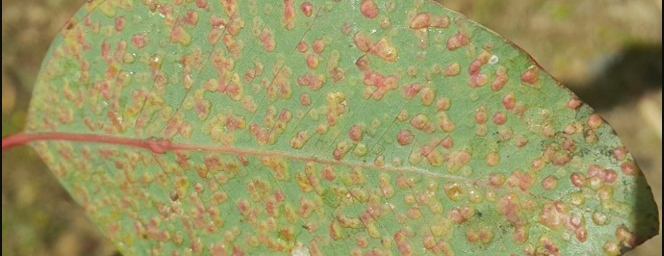
A serious canker disease of Eucalyptus species and hybrid clones, in the Zululand region of KwaZulu-Natal is caused by the fungus first known as Coniothyrium zuluense. Significant taxonomic revision of this pathogen and the family of fungi it belongs to has resulted in several name changes, with the most recent suggesting that it should be known as Teratosphaeria zuluense. It has also been shown that a second species, T. gauchensis occurs in South America and Ethiopia, causing a similar disease of eucalypts in those countries (Cortinas et al., 2006).
Coniothyrium canker is characterised by measle-like lesions on the young green stem tissue associated with the current year's growth. As the infection progresses, lesions become confluent and cracks that exude kino, develop in the necrotic tissue. Infection also results in severe malformation of the stems which become swollen and spindle-shaped in the areas of infection. New infections occur annually on new growth, while those from previous years continue to cause malformation and disease. Cankers, therefore, commonly occur in discrete zones at various heights on the stems of affected trees.
Susceptibility to Coniothyrium canker is variable on different clones. The most susceptible clones eventually cease to grow and their tops begin to die. It is unlikely that such clones can be successfully propagated commercially.
Pink disease of eucalypts
Erythricium salmonicolor (Corticium salmonicolor, Phanerochaeta salmonicolor) is a well-known canker pathogen of woody plants in tropical parts of the world and the associated disease is commonly known as pink disease. The disease has been known in South Africa for many years, particularly on deciduous fruit trees. It has been found affecting eucalypts, Acacia mearnsii and Podocarpus spp. in the KZN Midlands of South Africa, causing severe stem cankers and stem breakage on eucalypts and A. mearnsii. It has also been found causing branch and top death of Podocarpus spp. and Ekebergia capensis in the Sabie and Howick areas (Roux and Coetzee 2005).
Pink disease derives its name from the pink to salmon-coloured superficial fungal growth that occurs on the surface of cankers. Early in the infection process, a white mycelial mat develops on the bark surface. Cankers develop behind the leading edge of the mycelium where the cambium is penetrated by the fungus. The fungus requires relative humidity of more than 90 % for successful germination and infection to take place.
Cytospora (Valsa) canker of eucalypts
Three species of Cytospora, C. australiae, C. eucalypticola (Teleomorph: Valsa fabianae) and C. eucalyptina, have been reported associated with cankers on eucalypts in South Africa (Adams et al., 2005). In recent years, only C. eucalypticola has been found during routine surveys, and it has been shown that reports of C. australiae actually represent other species. Cytospora eucalypticola, which is also known on eucalypts elsewhere in the world, appears to be only mildly pathogenic. It is usually isolated from severely stressed trees and on dying tissue. Recent research has shown that the fungus is an endophyte that is present in asymptomatic tissue. It presumably then proliferates when stress conditions appear.
Bacterial wilt of eucalypts
Bacterial wilt of eucalypts is caused by the bacterium Ralstonia solanacearum. The disease is known in countries such as Brazil, China and Uganda and has been recorded from Eucalyptus clones in the Zululand forestry area of South Africa (Coutinho et al., 2000). The disease has, thus far, been found only in a small number of plantations where it has caused rapid wilt and death of trees at the onset of hot dry conditions in summer. Wilt is initially seen on single branches. Symptoms then spread rapidly to other branches and eventually the entire tree. The xylem of these trees show a brown discoloration and bacteria ooze from damaged wood almost immediately after cutting. When infected branches or stems are placed in water, a bacterial stream can easily be seen emerging from cut ends and the water soon becomes murky due to contamination with bacterial cells. There is strong emerging evidence to show that the bacterium is only serious on trees that are severely stressed and particularly those with root knots.
Black butt disease of wattle
Black discoloration of the bases of wattle trees is a common symptom wherever these trees are grown. The cause of this disease is complex and isolations from diseased tissue have yielded a number of opportunistic pathogens. It is likely that this symptom represents a complex disease situation without any single pathogen being responsible for the problem. It has also been suggested that gummosis at the bases of trees caused by Phytophthora nicotianae might be an initial stage of this disease, providing entry sites for opportunistic pathogens. Early infections by basidiomycetes and Phytophthora spp. might also provide entry wounds for the wilt pathogen, Ceratocystis albifundus, as well as other pathogens. This then leads to the spread of the black lesions to cover the entire tree in severe cases (Roux 2002).
Ceratocystis wilt of wattle
Ceratocystis wilt (wattle wilt) is caused by the fungus Ceratocystis albifundus and is the most serious disease of Acacia mearnsii in Africa. The disease occurs throughout South Africa, wherever A. mearnsii and A. decurrens occurs (Roux 2002).
Ceratocystis albifundus requires wounds to infect trees. Thus, wounds made when clearing plantations of weeds, or when stems of trees are 'singled' can lead to the onset of disease. Hail damage also provides excellent points of entry for C. albifundus and many other pathogens. Nitidulidae and other insects visiting wounds are the main means of spread of the pathogen. Once infection has occurred, the fungus moves rapidly within trees causing gum exudation from the stems. Wood becomes discoloured in a streaked pattern. Infe