Germination Tests
The International Seed Testing Association (ISTA) has a set of rules pertaining to the testing of germination of a range of plant species. These are contained in the International Rules for Seed Testing which is updated annually. According to ISTA, germination is defined as "the emergence and development of the seedling to a stage where the aspect of its essential structures indicates whether or not it is able to develop further into a satisfactory plant under favourable conditions in the soil". All the information to conduct a germination test for a particular crop is given in detail. This includes what substrate to use to plant the seeds, what temperature/s to incubate the planted seeds, what days to evaluate the seedlings and how to evaluate the seedlings. For example, Pinus radiata should be planted on top paper and incubated at 20°C, the first count to be made at 7 days and the final count to be made at 28 days. Pinus taeda, although also planted on top paper, needs to be incubated at 20 °C for 16 hours and 30 °C for 8 hours. The counts are made on the same days. An additional recommendation is to use a no prechill and a prechill of 28 days at 3 to 5 °C (double test). It is evident then from the above examples that a germination test is not merely placing seeds on blotter paper and then counting how many have emerged. Rules of how to evaluate normal and abnormal seedlings, hard, fresh and dead seeds are given and a handbook on germination with diagrams is available to help with these evaluations.
Germination test on germination paper



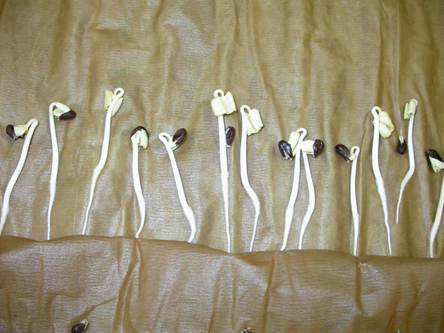
Growing media for the germination test
ISTA distinguishes among the following:
Paper substrates
|
Top of Paper (TP) Between paper (BP) Pleated paper (PP) |
Sand/Organic growing media
|
Top of sand (TS) Top of growing medium (TO) Sand (S) Organic growing medium (O) |
Paper and Sand
|
Top of paper covered with sand (TPS) |
Vigour Tests
According to ISTA seed vigour is "the sum of those properties that determine the activity and performance of seed lots of acceptable germination in a wide range of environments".Thus a vigorous seed lot should perform well even if environmental conditions are not optimal for growth of that specific species.
Vigour tests include:
|
The tetrazolium (TTZ) test The conductivity test The accelerated ageing test The cold test Rapid and slow imbibition test |
These tests provide additional information once the germination test has been done. These tests are usually not performed on seed that has a low germination potential. These tests also determine how seeds will perform if stored under sub-optimal conditions.
Seed Health Tests
Seeds may harbour pathogens both inside and on their surfaces. Seeds may be infected with viruses, bacteria, fungi and nematodes which may cause the seed to show various symptoms or may be dormant until the seedling emerges or until a certain plant age is reached. Some of these pathogens may be quarantine pests whilst others may give rise to epidemics. Most, however, are usually the cause of diseases being established in new fields or regions and/or may cause widespread disease to adjacent fields/crops when spores are dispersed from infected plants. ISTA has a range of tests that are used to test for seedborne pathogens. These tests have all been validated and are reviewed every 5 years. All new methods undergo an extensive validation procedure involving several laboratories worldwide before they are published in the ISTA Rules.